Araucariaceae
Araucaria araucana
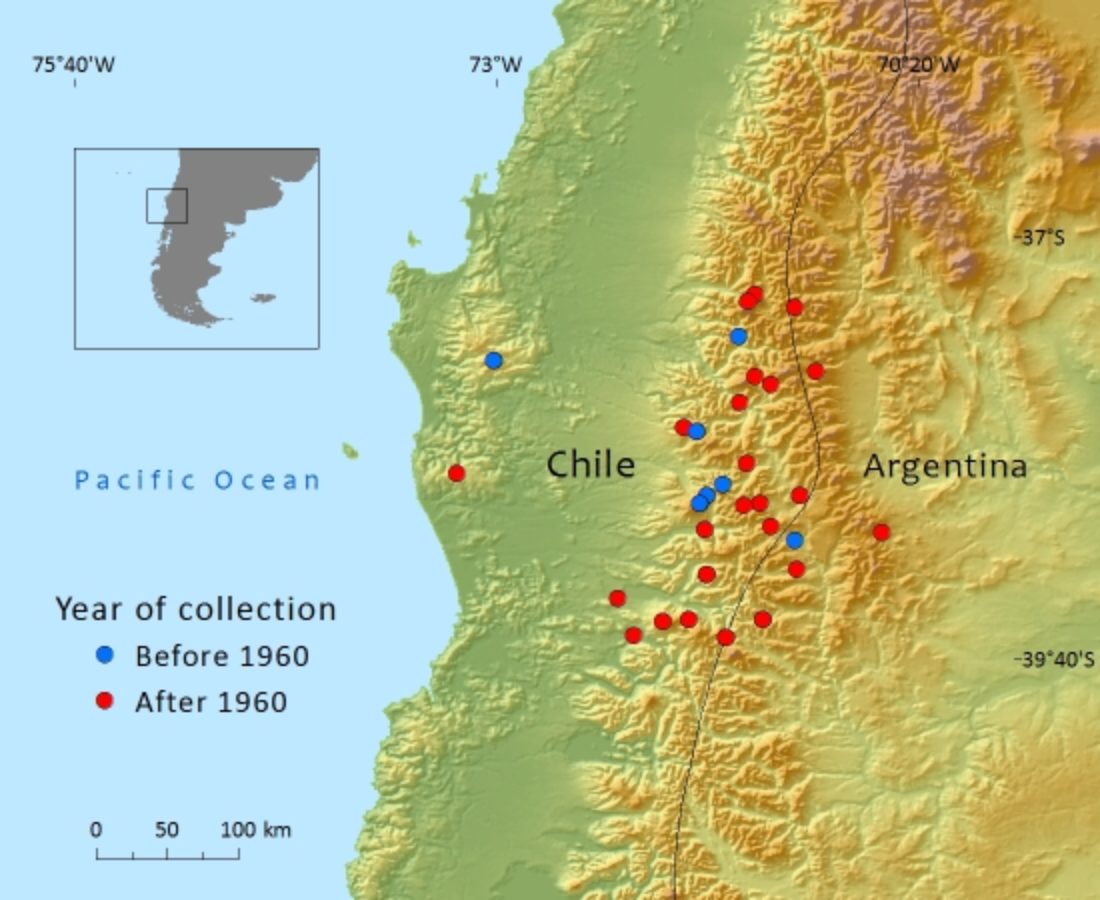
Endemic to southern Argentina and Chile where it mainly occurs in the Andes. Threats include fire, grazing and encroachment from commercial plantations of exotic species.
Description
Habit
Dioecious tree (rarely monecious) up to 50m tall; trunk to 2m in diameter; crown broadly pyramidal. Bark grey, usually deeply fissured and forming hexagonal plates. Branches vertical, horizontal or swept downwards, often restricted to the upper part of tree.
Foliage
Leaves 2.5-3 x 1.5–2cm, ovate-lanceolate, base broad, apex sharply pointed, sessile, coriaceous, rigid.
Cones
Male pollen-cones 8–12 x 4–5cm. Female seed-cones 15–20cm across, globose. Seeds 4-5 x 1.5cm, obconical-oblong or cuneiform, slightly compressed, with a large apical appendage, mid brown, 120–180 per cone; maturing from February to April (cones taking two years to mature).
Human Uses
Araucaria araucana has light, soft, medium-weight wood which is used for lumber, flooring, paper pulp and ship-masts (Delmastro & Donoso 1983). Due to its listing on Appendix I of CITES there is no legal international trade in the timber; currently there is only local use in Argentina and Chile. The seeds are very rich in carbohydrates and proteins and were once an essential part of the diet of the indigenous people; the seeds are boiled or roasted, and their taste is similar to that of chestnuts. Today, the seeds are still eaten by local inhabitants, both the indigenous people and settlers. It is also highly prized as an ornamental tree in Europe (especially throughout Great Britain) and in parts of North America.
References and further reading
- Aagesen, D.L. (1998). Indigenous rights and conservation of the monkey puzzle tree (Araucaria araucana Araucariaceae): a case study from Southern Chile). Economic Botany 52(2): 146-160.
- Aagesen, D.L. (1998). On the northern fringe of the South American temperate forest: The history and conservation of the monkey-puzzle tree. Environmental History 3(1): 64– 85.
- Anonymous. (2000). Proposal to Transfer from Appendix II to Appendix I of the Argentine population of monkey puzzle tree (Araucaria araucana), in accordance with the criteria specified in Resolution Conf. 9.2. Proposal 11.55 of the Eleventh Conference of the Parties. Gigiri (Kenya), 10-20 April 2000. 4 pp. Available at: http://www.cites.org/eng/cop/11/prop/55.pdf.
- Bekessy, S.A., Sleep, D., Stott, A., Menuccini, M., Thomas P., Ennos, R.A., Burgman M.A., Gardner, M.F. & A. C. Newton. (2002). Adaptation of Monkey Puzzle to Arid Environments Reflected by Regional Differences in Stable Carbon Isotope Ratio and Allocation to Root Biomass. Forest Genetics 9(1): 63-70.
- Bekessy, S, Lara, A, Gonzalez, M, Cortez, M, Gallo, L, Premoli, A and Newton, A (eds). (2004). Variación en Araucaria araucana. In: C. Donoso, A. Premoli, L. Gallo & R. Ipinza (eds), Variación Intraespecífica en las especies arbóreas de los bosques templados de Chile y Argentina, pp. 215-231. Editorial Universitaria.
- Bekessy, S.A., Sleep, D., Stott, A., Menuccini, M., Thomas P., Ennos, R.A., Burgman M.A., Gardner, M.F. & Newton, A.C. (2002). Adaptation of Monkey Puzzle to Arid Environments Reflected by Regional Differences in Stable Carbon Isotope Ratio and Allocation to Root Biomass. Forest Genetics 9(1): 63-70.
- Burns, B.R. (1993). Fire induced dynamics of Araucaria araucana - Nothofagus antarctica forest in the southern Andes. Journal of Biogeography 20: 669-685.
- CONAF, CONAMA, BIRF, Universidad Austral de Chile, Pontificia Universidad Católica de Chile, Universidad Católica de Temuco. (1999). Catastro y evaluatión de los Recursos Vegetacionales Nativos de Chile. Informe Nacional con Variables Ambientales. Santiago, Chile.
- Delmastro, R and Donoso, C. (1980). Review of distribution, variation and utilization of gene resources of Araucaria araucana (Mol.) Koch in Chile. Proc. IUFRO Symposio em melboramiento genetico e productividade de especias florestais de rapido crescimento
- Dodd, Z.A. and R.S. Dodd. (1998). Genetic diversity among coastal and Andean natural populations of Araucaria araucana (Molina) Koch. Biochemical Systematics & Ecology 26(4): 441-451.
- Echeverría, C., C. Zamorano & Cortés M. (2004). Conservation and restoration of Monkey Puzzle (Araucaria araucana) forests in Chile (Final Report).
- Finckh, M. and Paulsch, A. (1995). The ecological strategy of Araucaria araucana. Flora 190(365-382).
- Gallo, L., F. Izquierdo, L.J. Sanguinetti, A. Pinna, G. Siffredi, J. Ayesa, C. Lopez, A. Pelliza, N. Strizler, M. Gonzales Peñalba, L. Maresca & L. Chauchard. (2004). Araucaria araucana forest genetic resources in Argentina. In: Vinceti, B., W. Amaral and B. Meilleur (eds), Challenges in managing forest genetic resource for livelihoods: examples from Argentina and Brazil., pp. 105-131. International Plant Genetic Resources Institute, Rome.
- González, M.E., Veblen, T.T. & Sibold, S.(2005). Fire history of Araucaria -Nothofagus forests in Villarrica National Park, Chile. Journal of Biogeography 32: 1-15.
- Hampe, A. and Petit, R.J. (2005). Conserving biodiversity under climate change: the rear edge matters. Ecology Letters 8: 461-467.
- Hechenleitner, P., Gardner, M.F., Thomas, P.I., Echeverría, C., Escobar, B., Brownless, P. & Martínez, C. (2005). Plantas amenazadas del Centro-Sur de Chile. Distribución, Conservación y Propagación. Universidad Austral de Chile y Real Jardín Botánico de Edimburgo, Santiago.
- Marchelli, P. C. Baier, C. Mengel, B. Ziegenhagen & L. A. Gallo. (2010). Biogeographic history of the threatened species Araucaria araucana (Molina) K. Koch and implications for conservation: a case study with organelle DNA markers. Genetic conservation 11(3): 951-963.
- Peralta, M. (1980). Geomorfología, clima y suelos del tipo forestal Araucaria en Lonquimay. Facultad de Ciencias Forestales, Universidad de Chile, Santiago.
- Premoli, A., Quiroga, P. & Gardner, M. (2013). Araucaria araucana. The IUCN Red List of Threatened Species 2013: e.T31355A2805113. http://dx.doi.org/10.2305/IUCN.UK.2013-1.RLTS.T31355A2805113.en. Downloaded on 01 December 2014
- Rafii, Z.A. & Dodd, R.S. (1998). Genetic diversity among coastal and Andean natural populations of Araucaria araucana (Molina), K. Koch. Biochemical Systematic Ecology 26: 441-451.
- Schilling, R & Donoso, C. (1976). Reproduccion vegetativa natural de Araucaria araucana (Mol.) Koch. Investigaciones Agricultura (Chile), 2: 121-122.
- Veblen, T.T. (1982). Regeneration patterns in Araucaria araucana forests in Chile. Journal of Biogeography 9: 11-28.
- Veblen, T.T., Burns, B.R., Kitzberger, T., Lara, A. and Villalba, A. (1995). The Ecology of the Conifers of Southern South America. In: N.J. Enright and R.S. Hill (eds), Ecology of the Southern Conifers, pp. 129-135. Melbourne University Press, Carlton, Victoria.