Distribution
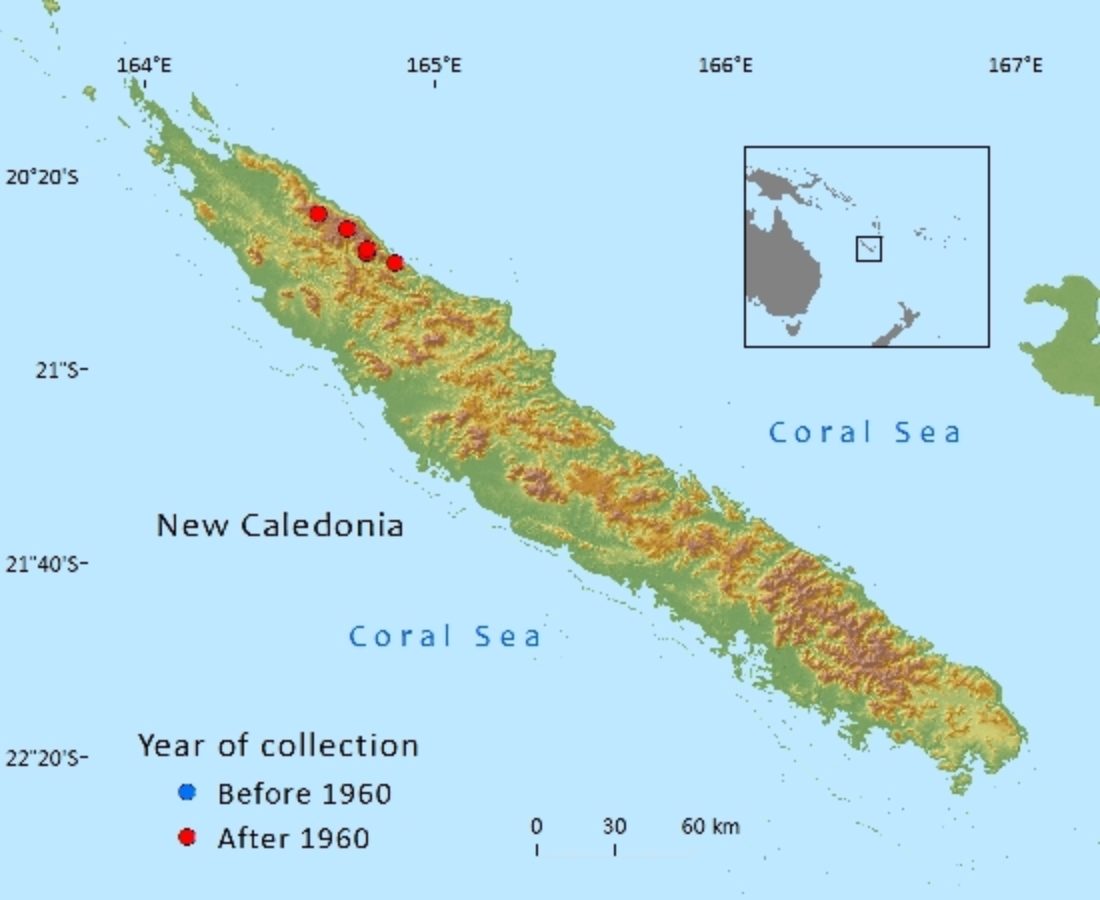
Endemic to New Caledonia in Province Nord where it is restricted to the Mt. Panié range. Its known locations are from the higher (above 1,000 m above sea level) altitudes of Mt. Panié, Mt. Colnett and Mt. Ignambi (Jaffré et al. ,1987). Agathis montana has also been recorded (MacKee 34469 (P), 22/12/1977) from the summit of the Roches de la Ouaième, a separate range facing Mt. Panié. This specimen was originally collected by J.-F. Cherrier but deposited under H.S. MacKee's collecting numbers. It was collected from a small tree at 950 m. Despite recent field botanical surveys (Munzinger, 2013) that included canopy surveys, no further observations or collections have been recorded from this locality, suggesting that this tree may be single and isolated. Local indigenous people have mentioned that this individual tree is well known to them and “has a story”, suggesting it may have been planted by their ancestors. There is thus some doubt about whether this tree should be considered as a natural occurrence. While the most recent guidance from IUCN on introduced subpopulations indicate that those that are geographically close to the known native range of the taxon being assessed should be included in any assessment, the guidelines also clearly state that such introductions must have produced viable offspring (IUCN Standards and Petitions Subcommittee 2014 p.7). As there is no indication of this, the Roches de la Ouaième locality is excluded from calculations of the extent of occurrence (EOO) and area of occupancy (AOO).
The overall EOO, (using herbarium records but excluding the single tree on the Roches de la Ouaième), is estimated to be 90 km2 with an estimated AOO of 32 km2. The estimate for the extent of occurrence uses coordinates taken from gazetteers to georeference older herbarium specimens. There is some uncertainty about how accurate the estimations are. The AOO is estimated using the standard IUCN recommended 2 km x 2 km grid – this is very likely to be an overestimate.
Agathis montana grows in mono-specific stands in the canopy above 1200 m on the north-eastern slope and above 1300 m on the south-western slope; all known trees seem to grow on slopes less than 35°. Using these data to model its range produces an estimated potential AOO of ca 1200 ha for the main population. When the few isolated trees growing down to 1000 m are included the estimate rises to ca 5,000 ha. Agathis montana forms a single subpopulation spread over a narrow altitudinal band on three peaks located within 20 km of each other along a single ridgeline.
Data on population size are based on 14 plots randomly established in October 2012 within 200 m of the Mt. Panié trail. Each plot is 50 m x 50 m, which represents 3.9% of the study area and 0.3% of the main A. montana range. Median density of trees >10 cm DBH (some of them may not yet be mature) is 83 trees/hectare (average = 104, min = 40, max = 356, standard deviation = 77) (Texier 2013). The total population may therefore be ca 100,000 mature trees. Based on 70 subplots of 10 m x 10 m designed to assess regeneration (saplings and young trees <10 cm DBH), median regeneration density is ca 3,000 individuals/hectare (average = 4,400, min = 0, max = 18,300) (Texier 2013).
A recent and apparently rapid decline in the population has been directly observed since at least 2009. It may have commenced somewhat earlier but the exact timing is uncertain. Photos taken by the New Caledonian botanist J.-M. Veillon in 1969 show few if any dead trees; those taken in 1996 by the herpetologist T. Whitaker do show some dead trees especially near the summit of Mt. Panié. Aerial photos taken in 1991, and studied by the main author of this assessment, appear to show some dead trees but the photos are of insufficient quality to allow a reliable quantitative analysis of the mortality at this date. Henri Blaffart, head of Mt. Panié conservation programme (2002-2008), and who visited the summit area many times during that period, did not report any kauri decline. Foreign botanists from various research organizations such as Institut de Recherche pour le Développement, Missouri Botanic Garden, the Royal Botanic Gardens Kew and the Royal Botanic Garden Edinburgh who also visited the area between 2002 and 2007 did not report any decline either. The previous IUCN assessment, published in 2010 but based on information collated up until 2007, stated that "Currently, no specific threats have been identified. Climate change impacts such as changes in precipitation patterns could be a problem in the future" (Thomas 2010).
A decline was first formally reported to the main author of this assessment in September 2009 by an indigenous local guide during a helicopter survey of the area (E. Vaiadimoin pers. comm.). Mt. Panié has always been a privileged but difficult destination for tourists and scientists, usually accompanied by local indigenous guides (four guides used to accompany ca 50 ascents per year in the late 2000s, (Anonyme 2011); these guides have thus an historical understanding over changes happening in this area. While this was the first formal record of the decline, they estimated in 2010 ‘it may have started some years ago’. Other members of the main local tribe have also reported that they had noticed a recent decline in the tree. In September-October 2012, 14 random plots were established along Mt. Panié trail (Texier 2013), containing 363 large trees (DBH >10 cm). Sixty seven of them (18.5%) were dead (health score = 6); of the live trees, 57 (19.2%) were showing significant die-back symptoms (health score = 4 or 5). In February 2014, 10 of those plots theoretically containing 257 trees were re-surveyed (Sabran and Tron 2014). Two hundred and thirty five were actually found and monitored; most of the 22 trees not found in 2014 are likely to have been overlooked because they might have fallen on the ground, considering their poor health in 2012 (10 were actually already dead). Out of the 235 monitored trees, 202 trees were alive in Oct 2012; amongst them, 10 were dead in February 2014, suggesting an annual mortality rate of 3.7% per year. Overall, 20.5% of monitored trees are now dead. While 42.6% of monitored live trees were assessed as healthy (health scores = 1 or 2) in October 2012, this percentage had declined to 15.6% in Feb 2014. Seven of the 10 trees that died between October 2012 and February 2014 had a health score of 3 or 4 in October 2012. Forty four (27.2%) of the live trees now have a health score that represents a relative decline of two or more points over the monitoring period. These data suggest a significant and rapid decline.
Using the available data (of the 192 live trees remaining in our monitoring programme, 10 are projected to die every 16 months), an 80% population reduction of trees >10 cm DBH would happen within 21 years. Using population structure data from 2012 survey, we estimate nine new trees from the regeneration cohort would reach 10 cm DBH within the next 21 years: this is insufficient to replace the losses. Within 100 years (which is way below three generation lengths) there would be no mature trees left. Considering the significant reduction of many tree's health between October 2012 and February 2014 and sampling/monitoring biases (see below) resulting in an underestimation of mortality rate, the decline might be faster. These projections are based on limited data recorded over a relatively short period of time.
Notes on sampling and data reliability:
Helicopter flights in 2009 and 2011, as well as preliminary 2012 aerial images analysis, suggest that mortality is significant over the entire southwestern slope and all along the main ridgeline. The same approach suggests that lower altitude stands (i.e. between 1200 and 1300 m) on the northeastern slope are less affected. The four non monitored plots had – in October 2012 - a higher mortality rate, suggesting the proportion of dead trees may be higher over the entire 2012 study area: most of the 12 live trees from October 2012 that were not found in February 2014 are likely to be dead considering their poor health in October 2012, suggesting the mortality rate quoted here may actually be higher than 3.7% per year. Based on the information presented here the species could qualify for a threatened listing based on criterion A4bce, however, due to the small sample size of plants in the monitoring area and the limited time period for which the situation has been monitored, it was decided that there was too much uncertainty about the long-term trend to use this information in the assessment of this species.
Habitat and Ecology
Most trees grow in mono-specific stands in the canopy above 1300 m on the SW slope and above 1200 m on the NE slope. Below that, only a few isolated individual trees are seen down to 1000 m (de Laubenfels, 1972). This elevation range seems to correspond to a cloud layer that envelopes this part of the mountain most of the time.
Agathis montana forms near-monospecific stands that dominate the canopy between 10 and 25 meters above ground. It probably plays a significant role (=keystone species) in filtering sunlight and maintaining humidity and thus creating a unique habitat. Most other plant species grow up to 3-5 meters, creating a very thick understorey (Tron and Texier 2013). Several micro-endemic plant species (Wulff 2013) and insects (Grandcolas et al. 2008) are known from this habitat, one of the most important for micro-endemism in New Caledonia (Wulff 2013) and now internationally recognized as a "Key Biodiversity Area" (Conservation International 2011).
One recently dead tree of 80 cm diameter was estimated to be aged 1,100-1,300 years based on Carbon 14 dating methods (GNS Science 2012). Life-span is therefore suspected to be well over 1,000 years (Tron and Texier 2013). Based on this information and assuming linear growth, annual diameter growth is expected to be 0.7 mm/year. Fruiting is irregular and few observation have been made; however, maturity may be reached when trees are 20-30 cm DBH. Generation length may be ca 500 years. Regeneration is very dense in the understorey, but very few of them reach maturity (Texier 2013). From casual observations, regeneration looks sparse or may even be absent when mature A. montana are dead: most dead trees are in eroded areas.
Agathis montana produces a dense, fine and highly ectomycorhized feeder-roots matt within the first 5 cm of the soil (Tron and Texier 2013).The soil is silty and shallow (<50 cm) and seems to be the product of the bedrock (schist) and litter degradation. It is therefore particularly sensitive to erosion. It does not grow on purely rocky substrate outcrops.
The population dynamics of the family Araucariaceae are thought to involve, or even be dependent on severe landscape scale perturbations like cyclones or bushfires (Enright et al. 1999). This may explain some of the results of the original population structure surveys from 2012 (Texier 2013).
The indigenous organization in charge of the management of Mt. Panié wilderness reserve, is named after A. montana. They hence have a key cultural responsibility in caring for that species in particular. Agathis montana is an iconic species, often displayed on pictures promoting New Caledonian biodiversity and landscape.